Which Ions Have the Same Electron Configuration as Argon
This phenomenon is called ion-molecule reactions. As it needs only one electron in its valence shell to complete the octet and attain the noble gas configuration of Argon left 288 right it readily accepts the electron given by the sodium atom.

Electron Configuration For Ti Ti3 And Ti4 Titanium And Titanium I Electron Configuration Electrons Educational Videos
Monatomic ions are atoms that have either lost for cations or gained for anions electrons.

. The chlorine atom which has a high electronegativity gains an electron and is converted into a chloride ion that has the same electron configuration as argon ls 2 2s 2 2p 6 3s 2 3p 6. Lewis electron dot diagrams for ions have fewer for cations or more for anions dots than the corresponding atom. Chemical ionization technique uses virtually the same ion source device as in electron impact except CI uses tight ion source and reagent gas.
Its easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. The sodium ion is isoelectronic with the neon atom. 1s is the closest and lowest energy orbital to the nucleus.
The electron configuration of the sodium ion is now the same as that of the noble gas neon. These elements already have a full outer energy level so they are very stable. Consider a similar process with magnesium and with aluminum.
Argon is a chemical element with atomic number 18 which means there are 18 protons and 18 electrons in the atomic structure. 35 minus 18 is 17 so you would write argon in brackets and follow it with 4s2. For each electron shell atom diagram the element symbol is listed in the nucleus.
As such noble gases typically do not react with other elements to form compounds. The valence electron configuration of thallium. Helium He Neon Ne Argon Ar Krypton Kr Xenon Xe Radon Rn element 118 oganesson Og 5 Properties Of Nonmetals 1.
Argon is the third-most abundant gas in the Earths atmosphere at 0934 9340 ppmv. The electron configuration of potassium-ion shows that potassium ions have three shells and the last shell has eight electrons. Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol.
And Paulis exclusion principle is that the value of four quantum numbers of two electrons in an atom cannot be the same. For that we have electron shell diagrams. Sample ions are formed by the interaction of reagent gas ions and sample molecules.
The chemical symbol for Argon is Ar. The chloride ion has a 1 charge because there are 17 protons in the nucleus but there are 18 electrons about the nucleus of the ion. The term isoelectronic refers to an atom and an ion of a different atom or two different ions that have the same electron configuration.
The electron configuration shows that the potassium atom has acquired the electron configuration of argon and it achieves a stable electron. Therefore the electron will first enter the 1s orbital. Electron dot diagrams for ions are the same as.
The electron configuration of potassium ionK is 1s 2 2s 2 2p 6 3s 2 3p 6. The electron configuration of the sodium ion is now the same as that of the noble gas neon. The formation of sodium chloride from the sodium and.
The noble gases are. Bromine has 35 electrons. Here are electron shell atom diagrams for the elements ordered by increasing atomic number.
Atoms of group 18 elements have eight valence electrons or two in the case of helium. Which has been discussed in detail above. Noble gases have a full valence shell of 8 electrons so they exist in a very stable electron configuration.
Argon is mostly used as an inert shielding gas in welding and other high-temperature industrial. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration. Reagent gas molecules are.
Ammonia is first subjected to electron impact. You would do the same with bromine as argon is also its immediately preceding noble gas. The sodium ion is isoelectronic with the neon atom.
To write the orbital diagram of chromiumCr you have to do the electron configuration of chromium. Explain why the first two dots in a Lewis electron dot diagram are drawn on the. Thus the electron dot diagrams for the first column of elements are as follows.
The term isoelectronic refers to an atom and an ion of a different atom or two different ions that have the same electron configuration.

Inorganic Chemistry 9781423214311 In 2022 Chemistry Study Guide Chemistry Lessons Chemistry Education

Sezen Kocabiyik Et 90 Autres Utilisateurs Ont Enregistre 81 De Vos Epingles Chemistry Classroom Chemistry Lessons Chemistry Education

Electron Configurations Ck 12 Foundation

Electron Configuration Worksheet Answers Part A Worksheets For Chemistry Worksheets Electron Configuration Matter Worksheets

Electron Distribution Worksheets Answers Chemistry Worksheets Electron Configuration Chemistry Lessons

The Properties Of The Noble Inert Gases Inert Gas Noble Gas Ionization Energy
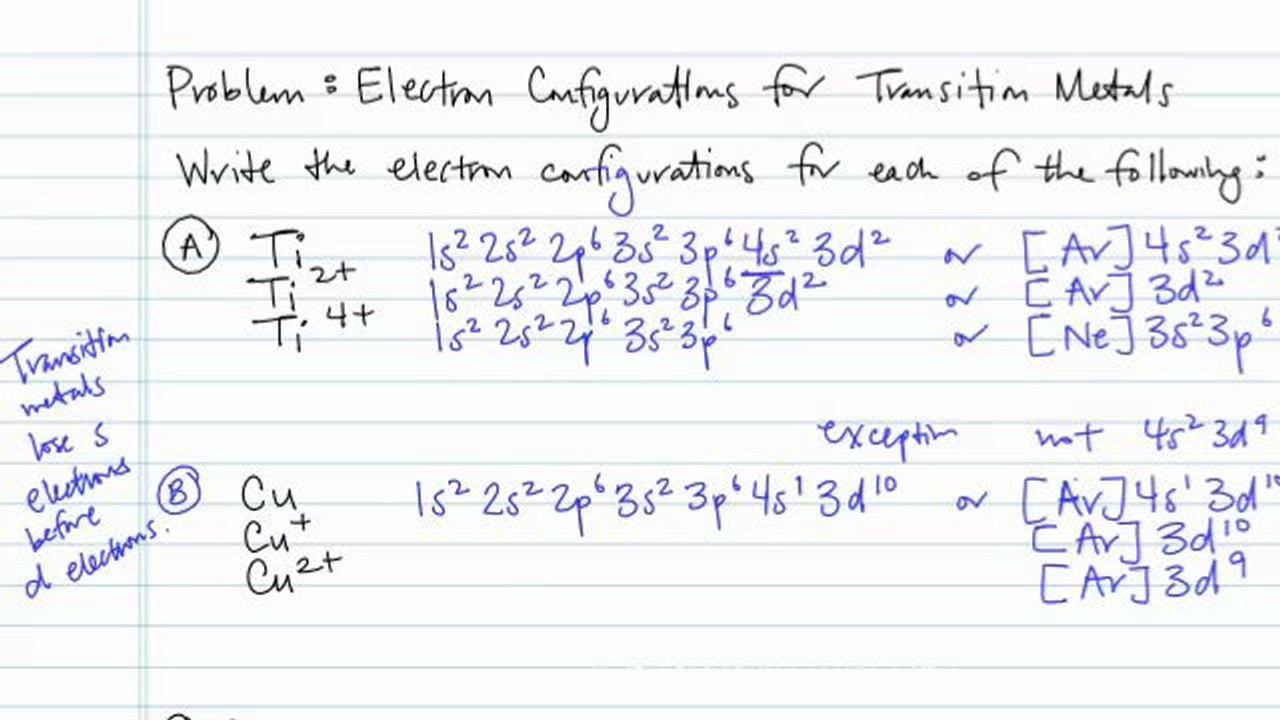
Electron Configurations For Transition Metals And Their Ions Problem Concept Chemistry Video By Brightstorm

Kerala Syllabus 10th Standard Chemistry Solutions Chapter 1 Periodic Table And Electronic Configuration Electron Configuration Chemistry Basics Chemistry Help

What Are The First 20 Elements Periodic Table Of The Elements Periodic Table Words Periodic Table Art

See The Electron Configuration Diagrams For Atoms Of The Elements Potassium Atom Atom Diagram Electron Configuration
What Is The Electron Configuration Of Sc Quora
Electron Configurations Of Ions Video Khan Academy

Electron Configuration Of Ions Mg2 P3 Fe2 Fe3 Youtube

Electron Configuration For Argon Ar

Atoms Isotopes And Ions Kit Electron Configuration Teacher Guides Curriculum Development

Electron Configurations Ck 12 Foundation
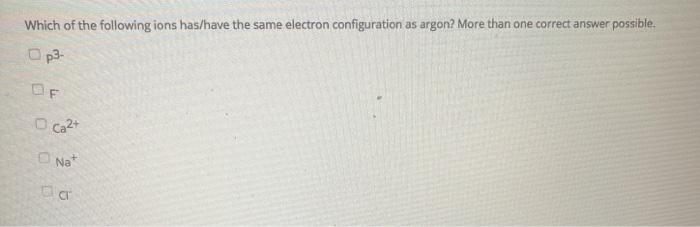
Solved Which Of The Following Ions Has Have The Same Chegg Com
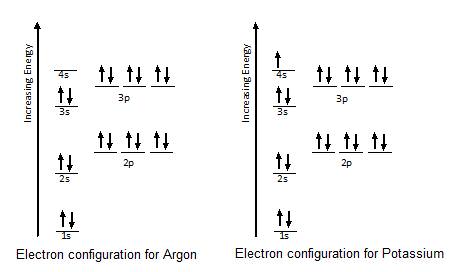
Electron Configurations Ck 12 Foundation

6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
Comments
Post a Comment